Facilities and Services
Click the button below to inquire about services, partnerships and business development opportunities.


Our CL2 laboratory supports clinical trials, serves as a referral lab for industry and collaborative research, and operates as an independently funded facility specializing in infectious diseases and immunology.
Located in a restricted access wing on the third floor of the IWK Goldbloom Research Centre, the CCfV Lab Operations Team oversees all stages of research projects, from start-up to close-out and archival.

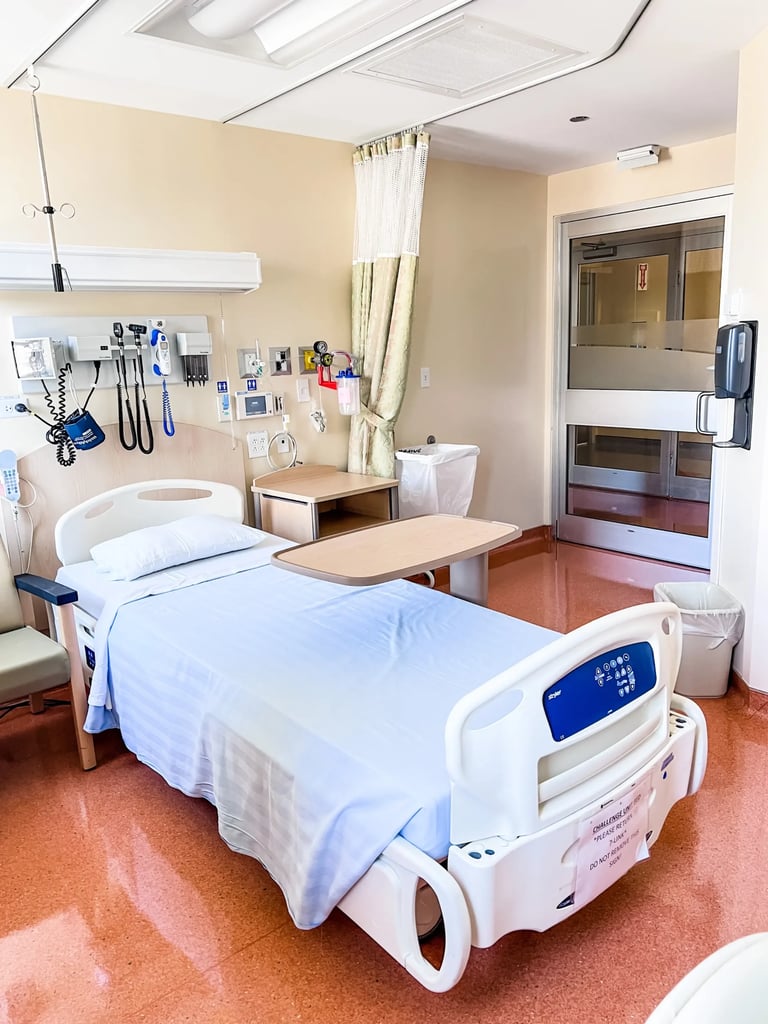
Our Challenge Unit is a cutting-edge facility designed for inpatient and outpatient research. It features ten single-bed isolation rooms with full monitoring capabilities, negative air pressure, and HEPA-filtered exhaust to handle respiratory pathogens safely.

Our Data Management and Analysis team is dedicated to maintaining high data quality standards and detailed study timelines.
The team ensures compliance with all regulations and guidelines, adhering to high data quality standards and Good Clinical Data Management Practice (GCDMP) throughout the research process.
All data analysis performed at CCfV is under the direction of the statistical investigator, who is responsible for the design, execution, and reporting of statistical analyses based on the requirements of individual projects.
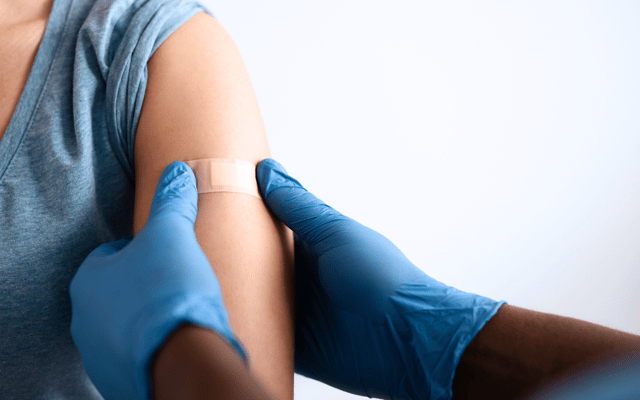
Clinical researchers have access to full-spectrum infrastructure and resources needed to conduct clinical research including nursing support for inpatient and outpatient data collection, protocol-specific interventions, and all other trial-related activities.
Our comprehensive clinical services encompass recruitment, staffing, equipment, laboratory, investigational product management, and facilitating monitoring support.

CCfV has been at the forefront of vaccine related research since 1992, conducting hundreds of academic and industry-sponsored studies across Phases 1-4.
As a collaborative, multidisciplinary research centre, we have expertise and capabilities to undertake research related to vaccines and infectious disease such as epidemiology, surveillance, immunology and more.
At CCfV, we combine cutting-edge infrastructure with unparalleled expertise to deliver exceptional outcomes in vaccinology research. Join us in shaping the future of healthcare.
Contact
Sedric Pankras
Senior Manager, Business Development
sedric.pankras@dal.ca

CCfV welcomes academic, industry, non-profit and government partners and collaborators at its facilities. Pending a request for access from any potential user, CCfV’s Operations Committee reviews the request for feasibility, scientific merit, financial viability, and risk to ensure that projects reflect the organization’s commitment to equity, diversity, and inclusivity. Priority access is provided to Canadian academic, government, and NGO users, followed by for-profit or industry users. Projects focused on research that may benefit underserved or special populations are also given a higher priority; recently, CCfV successfully collaborated on multiple projects with Indigenous communities (Nunavut, Nunatsiavut, Mi’kma’ki); Canadians of African descent, and international populations in developing countries (Bangladesh, Burkina Faso, Senegal).
For projects led or initiated by academic, publicly funded, or non-profit users, CCfV develops at-cost budgets with preferential pricing. CCfV budgets are based on a cost-plus margin for industry and for-profit groups. A full feasibility assessment is conducted once the Operations Committee determines that the requests meet CCfV’s standards and strategic goals. The user request then undergoes protocol/project review by a Principal Investigator; protocol, budget and deliverable review by the CAO and Associate Directors to assess feasibility and determine risks; and contract or agreement review in association with legal counsel to assess institutional and investigator risk and responsibilities.
Pricing is based on several factors and is developed through a three-tiered process. An initial high-level quote can be provided based on the estimated service requirements, with a more detailed budget to follow review of an executive summary of the proposed activities and/or projects. A final budget will then be developed based on the finalized protocol or statement of work, to be agreed upon by the Scientific Director, CCfV, Chief Financial Officer, CCfV, and potential collaborator(s).